More than 20 years of experience in project management roles with + 200 (sub-) projects. Many years of management and management consulting practice in the healthcare industry with a focus on process optimization, strategy & business development, transformation, competence development, sustainability and regulation


MedTech & LifeSciences
At the intersection of innovation, transformation, compliance and sustainability.
MedTech & LifeSciences at CBR combine technical, regulatory, and commercial expertise to support health and science driven organisations.
Our Industry Focus
MedTech & LifeSciences span highly regulated, innovation-driven sectors – from medical devices and diagnostics to biopharma, healthcare systems, and digital health.
At CBR, we harness this complex and interconnected ecosystem to deliver strategic, measurable impact across the full value chain — from R&D and manufacturing to market access, from compliance and digitalisation to sustainability.
See where we drive transformation across the MedTech & Life Sciences landscape:
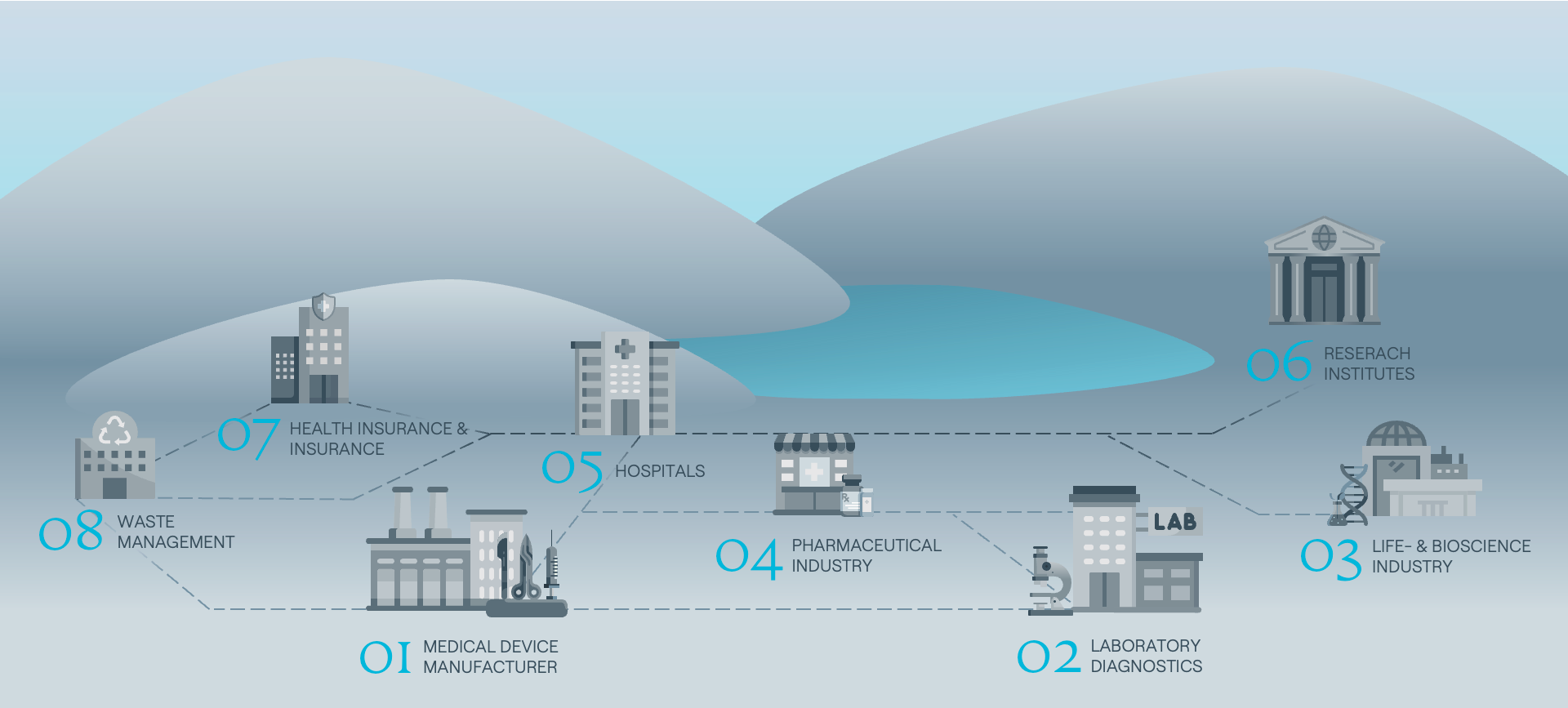
01 Medical Device Manufacturer
Supporting medical device manufacturers with process efficiency optimization, digital compliance solutions and circular product innovation to future-proof portfolios in a tightly regulated environment.
02 Laboratory Diagnostics
Helping diagnostics companies manage regulatory transitions, digitise product information flows and implement sustainability across the lifecycle of reagents, consumables and equipment.
03 Life- & Biosciences
Enabling bioscience and life science companies to embed resilience strategies, manage product and data compliance and prepare for circular innovation across research, production and commercialisation.
04 Pharmaceutical Industry
Advising pharma clients on sustainable product strategies, supply chain due diligence and decarbonisation, while ensuring regulatory alignment and operational continuity in global markets.
05 Hospitals
Supporting healthcare providers in optimising resource efficiency, navigating ESG reporting obligations and building resilience through sustainable infrastructure and compliance systems.
06 Research Institutes
Partnering with public and private research institutions to align complex transformational projects with sustainability frameworks, funding strategies and regulatory readiness in highly specialized environments.
07 Health Insurance & Insurance
Helping insurers integrate sustainability into risk modelling, governance and transparency, while navigating CSRD, taxonomy and social impact regulations in the health economy.
08 Waste Management & Recycling Industry
Securing the valuable medical waste resources as feedstock for other industries and establishing circular and waste management activities in the healthcare sector.
Trusted by Industry Leaders
MedTech & LifeSciences Insights
Contact Our MedTech & LifeScience Experts

Frank Roth
Principal
With over two decades in healthcare- and project-management roles, on the corporate side and as a management consultant, I am committed to driving business development, innovation and sustainable strategies. My focus lies in enhancing regulatory compliance and developing business models that adapt to evolving environmental, social, and governance obligations.

Frank Roth
Principal
Professional Experience
Consulting Focus @ CBR
Regulatory, commercial and technological expertise in the healthcare industry
Development and implementation of sustainable business strategies in the course of existing and upcoming “ESG obligations”
Implementation of regulatory frameworks and ensuring of compliance (EU taxonomy, CSRD, LkSG, net-zero approaches, green energy and raw materials requirements, etc.)
Education
EMBA -Executive Master of Business Administration-, Kellogg School of Management at Northwestern University / WHU Otto Beisheim School of Management
Dipl.-Ing. (FH) – Biomedical Engineering, University of Applied Sciences Gießen
Certified business trainer & coach (dvct standard)

Andreas Egloff
Project Manager
Professional experience
More than five years of experience in multinational, multi-site and interdisciplinary projects in MedTech, especially in production, process validation, equipment qualification and implementation of the European Medical Device Regulatory.
Consulting focus @ CBR
Life Science, Medtech and Sustainability
Circular economy in healthcare including new products and business models
European Medical Device Regulation with focus on manufacturing
German Supply Chain Due Diligence Act
Education
Master of Business Administration (MBA) in Sustainability Management, Leuphana University, Lüneburg, Germany
Master in Mechanical Engineering, Karlsruhe Institute of Technology, Karlsruhe, Germany / University of Zagreb, Croatia
Bachelor in Mechanical Engineering, Karlsruhe Institute of Technology, Karlsruhe, Germany
We look forward to discussing how we can navigate the path to success together.
Deichstraße 29 | 20459 Hamburg | Germany
Email: info@cbr-partner.de
phone: +49 (0) 40 3093 3850
mobile: +49 (0) 152 2593 6018













